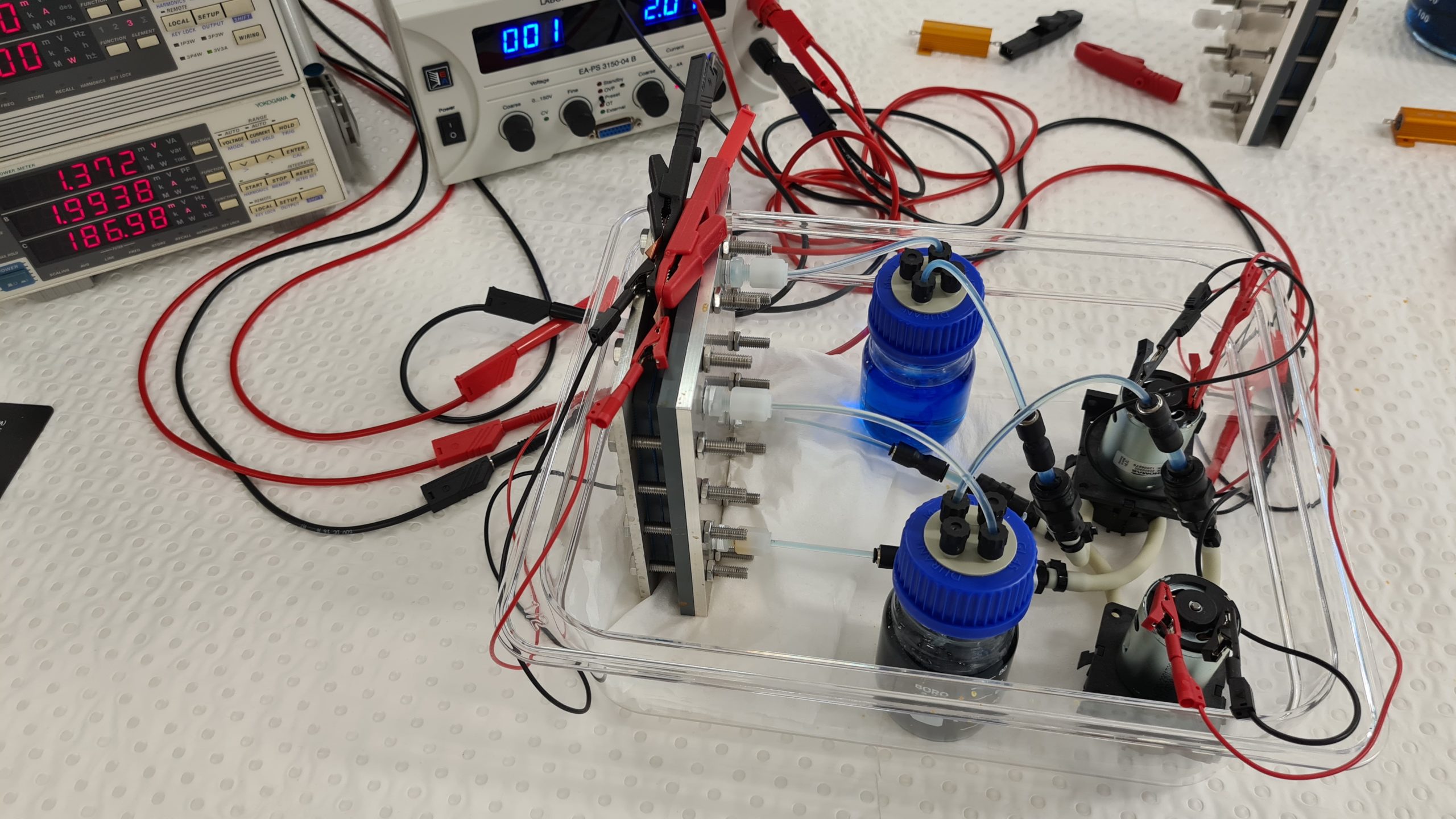
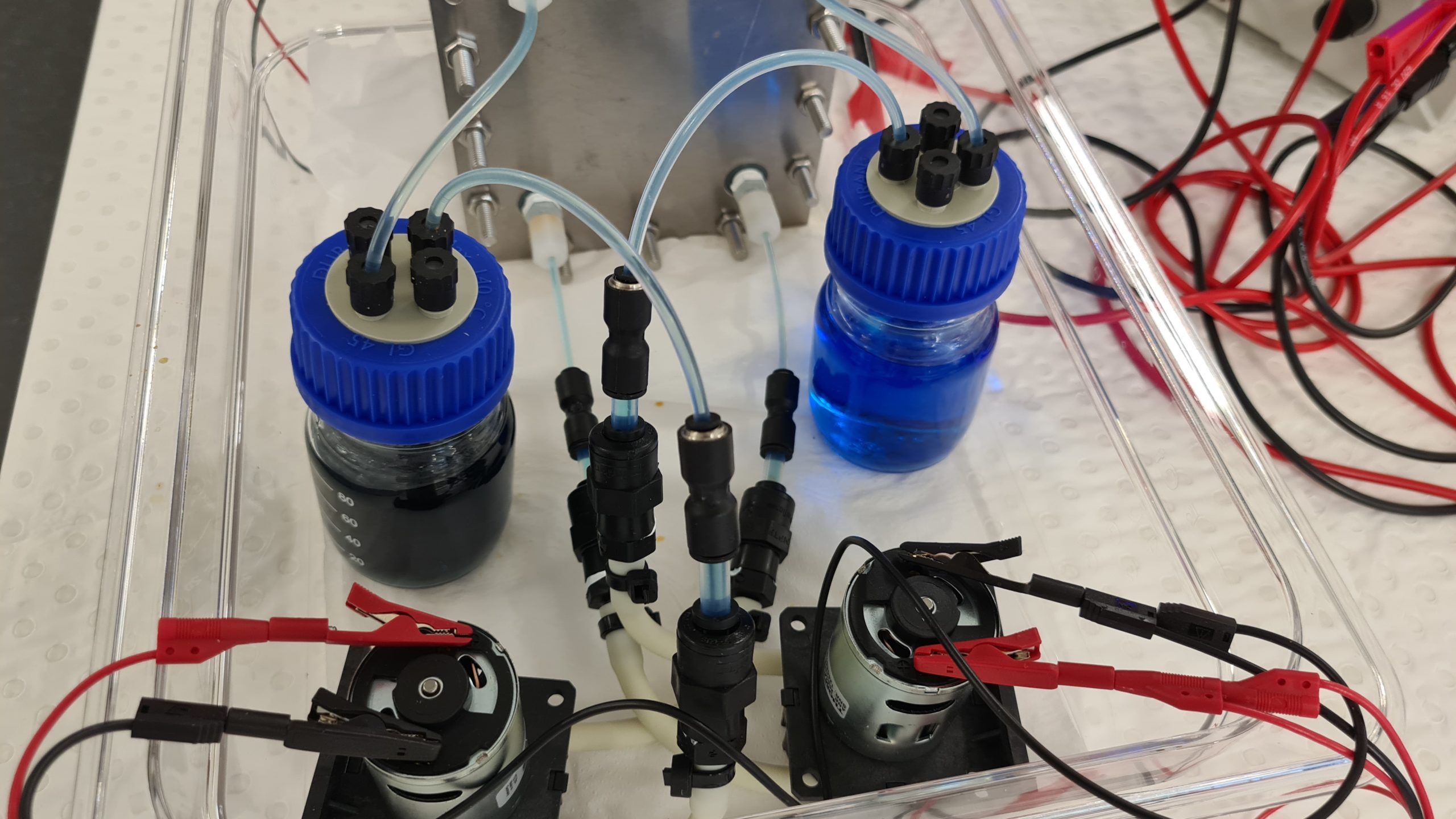
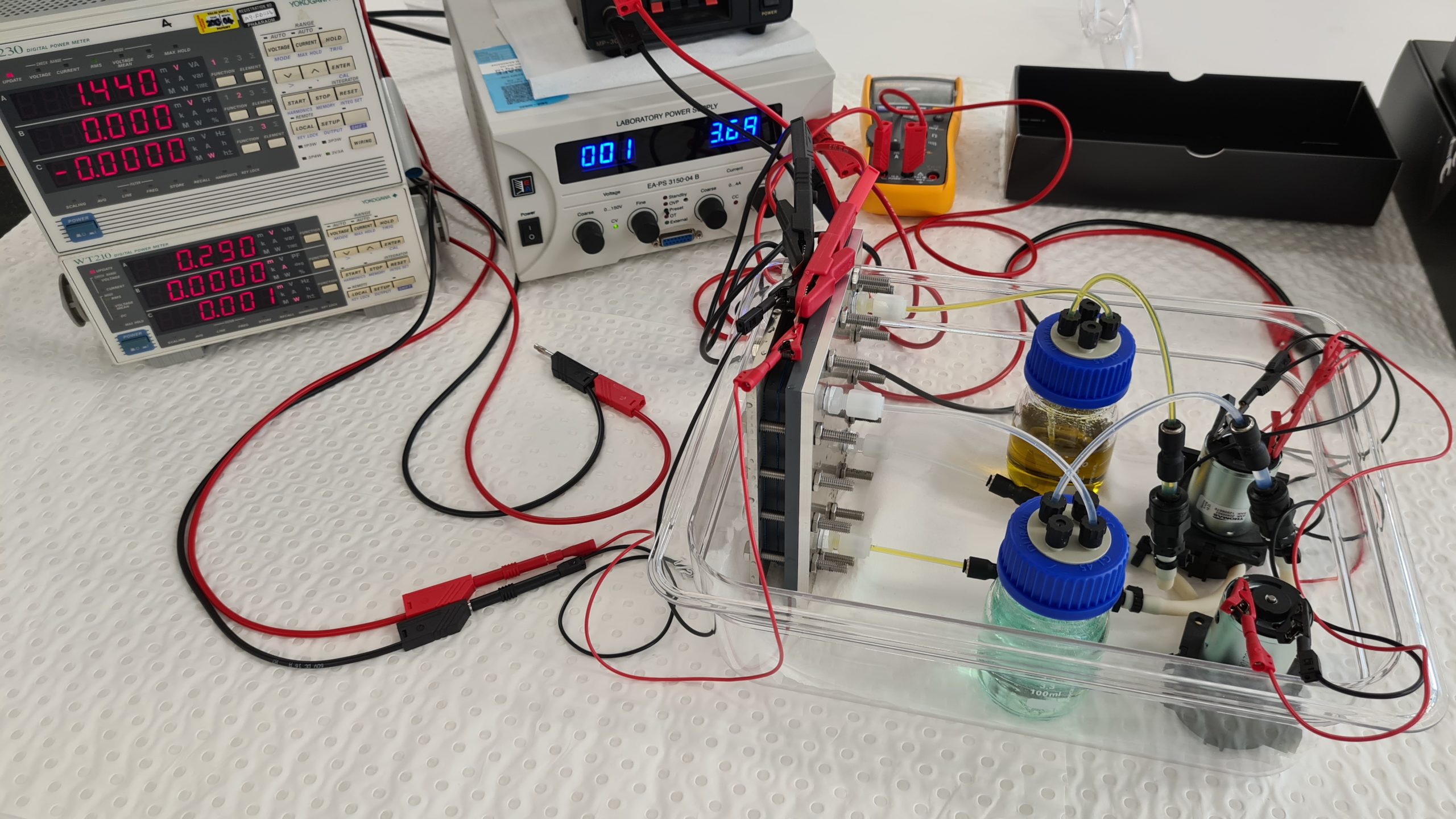
This is what a vanadium redox flow battery looks like for teaching purposes. We use a very diluted vanadium solution here, on the one hand to make the four color transitions visible and on the other hand to keep the charging and discharging times low. The battery here has a very low energy density, but can be fully charged in 15 minutes. Yellow and purple colored vanadium solutions show a fully charged battery, green and blue solutions a fully discharged battery.