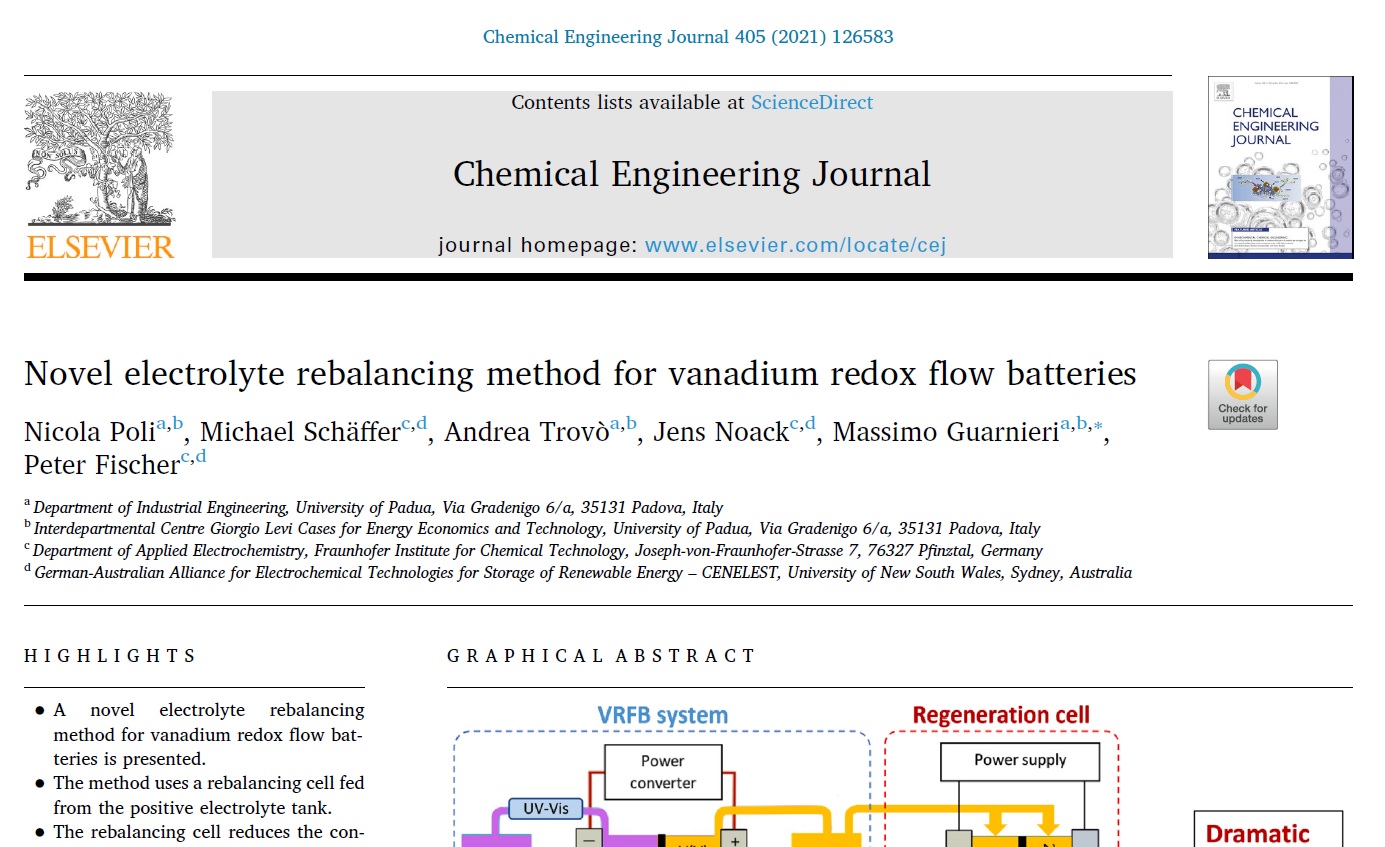
For almost three years now, a collaboration with the University of Padua in the field of redox flow batteries has existed with the Fraunhofer ICT. It is carried out through the Erasmus Mundus scholarship program between the group of Prof. Massimo Guarnieri at the University of Padua and the Department of Applied Electrochemistry. The results of the last master thesis in this collaboration of Mr. Nicola Poli have now been published in the prestigious Chemical Engineering Journal (Impact Factor 10.652).
Based on a patent of the Fraunhofer ICT the application of so-called regeneration cells was investigated. With the help of these cells, irreversible changes in the electrolyte, which lead to a loss of capacity, can be electrochemically compensated. One of these possible changes is the entry of oxygen, which can diffuse through the plastic parts of the battery. This entry can oxidize vanadium and thus lead to a permanent loss of capacity in the long run. With the help of a small extra cell, which produces oxygen and reduces the electrolyte again, this oxidation can be reversed and the state of charge of the electrolyte can be balanced again.
Such a cell can help to maintain the state of charge of a battery in the long term and make electrolyte maintenance unnecessary. The difficulty of the work was to achieve an end point determination of the charge balance without additional sensor technology. Nicola Poli could prove that this is possible by controlling the charge current. https://www.sciencedirect.com/science/article/abs/pii/S138589472032711X
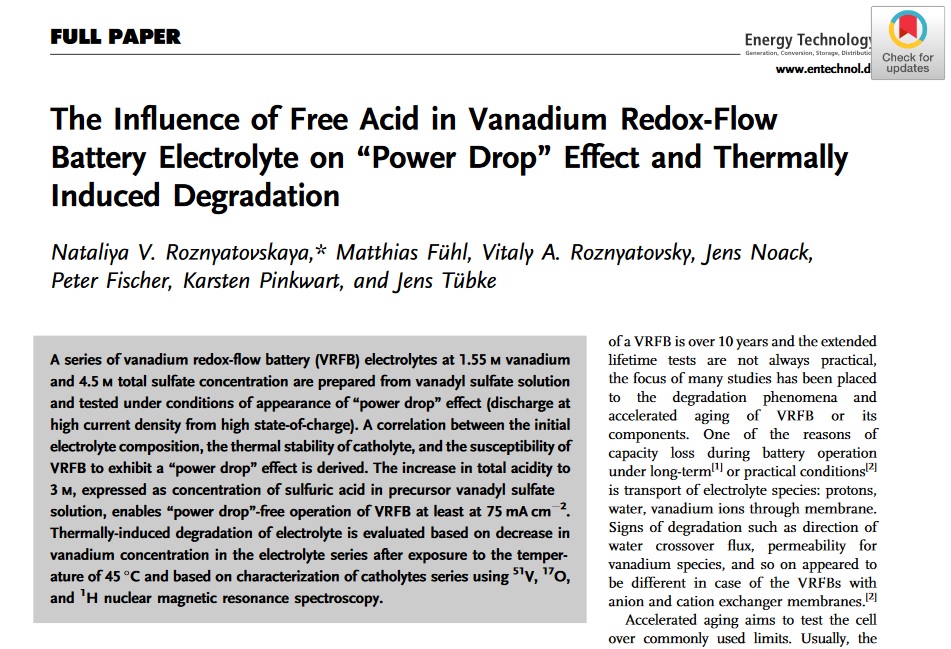
New paper about the “power drop effect” in vanadium redox flow batteries
Nataliya Roznyatovskaya et al. published a new paper on studies about the “power drop effect” in vanadium redox flow batteries. Free acid content in electrolytes for vanadium redox-flow batteries is a hardly accessible parameter in practice. If it can be roughly linked to electrolyte conductivity within the series of electrolytes with varied free acid content, such reversible or irreversible degradation phenomena as „power drop“ effect during discharge and thermally-induced aging of catholyte are shown to be dependent on electrolyte acidity. https://onlinelibrary.wiley.com/doi/full/10.1002/ente.202000445
Understanding vanadium redox flow batteries
Members of CENELEST have for the second time written an article for PV-Tech Power about redox flow batteries. While the first part was about redox flow batteries in general, the second part is specifically about vanadium redox flow batteries. The complete journal can be downloaded here after registration: https://store.pv-tech.org/store/pv-tech-power-volume-23/
Redox flow batteries for renewable energy storage
As energy storage becomes an increasingly integral part of a renewables-based electricity system, new technologies are coming to the fore. Jens Noack, Nataliya Roznyatovskaya, Chris Menictas and Maria Skyllas-Kazacos from CENELEST, a joint research venture between the Fraunhofer Institute for Chemical Technology and the University of New South Wales, chart the rise of redox flow batteries, a promising alternative to lithium-ion-based systems.
The full length article is available at PV Tech: https://store.pv-tech.org/store/redox-flow-batteries-for-renewable-energy-storage/
The German International Bureau rated CENELEST/Zenith as successful
The International Bureau is a part of the German project management agency DLR-PT and responsible for CENELEST. Via DLR-PT CENELEST is recieving funding from the German Ministry for Education and Research (BMBF) to establish this international collaboration between Germany and Australia for the years 2017-2022. All those involved in establishing the joint research presence thank the help of the International Bureau, DLR-PT and BMBF and are looking forward to further fruitful cooperation and excellent results through CENELEST.