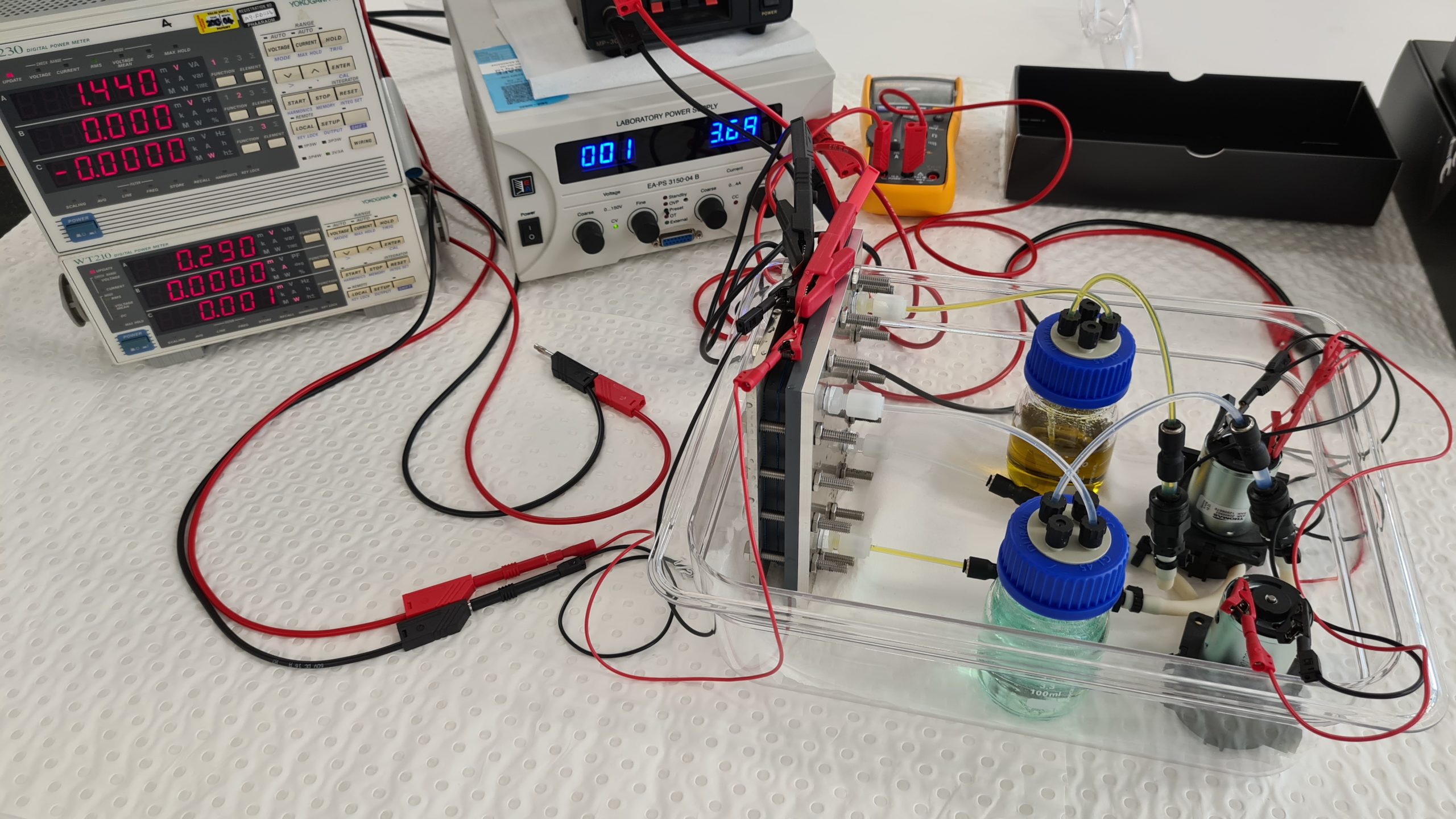
Here is an iron/iron redox flow battery set up for educational purposes. A Fe/Fe-RFB uses a green Fe(II) salt solution as the initial solution in the discharged state, whereby the solution is only slightly acidic. During the charging process, more brown iron(III) ions are formed in the positive solution. The negative solution becomes paler because the concentration of iron(II) ions in the solution decreases with the state of charge due to the deposition of elemental iron. As a result, the state of charge can also be detected very well here.