I visited today Dr. Depan Kundu’s Lab for Battery Research and Innovation at UNSW. We discussed research on sodium ion and solid state batteries.
Talk at the TU-Dresden 6th Autumn Workshop on Energy Storage
I gave an invited talk on the current state of redox flow batteries at the 6th Autumn Workshop on Energy Storage at TU-Dresden on November 30.
You can download the full presentation here:
Jens Noack – Status and perspectives of current flow battery technologies
Setting up a vanadium redox flow battery (VRFB) experiment for teaching at UNSW
This is what a vanadium redox flow battery looks like for teaching purposes. We use a very diluted vanadium solution here, on the one hand to make the four color transitions visible and on the other hand to keep the charging and discharging times low. The battery here has a very low energy density, but can be fully charged in 15 minutes. Yellow and purple colored vanadium solutions show a fully charged battery, green and blue solutions a fully discharged battery.
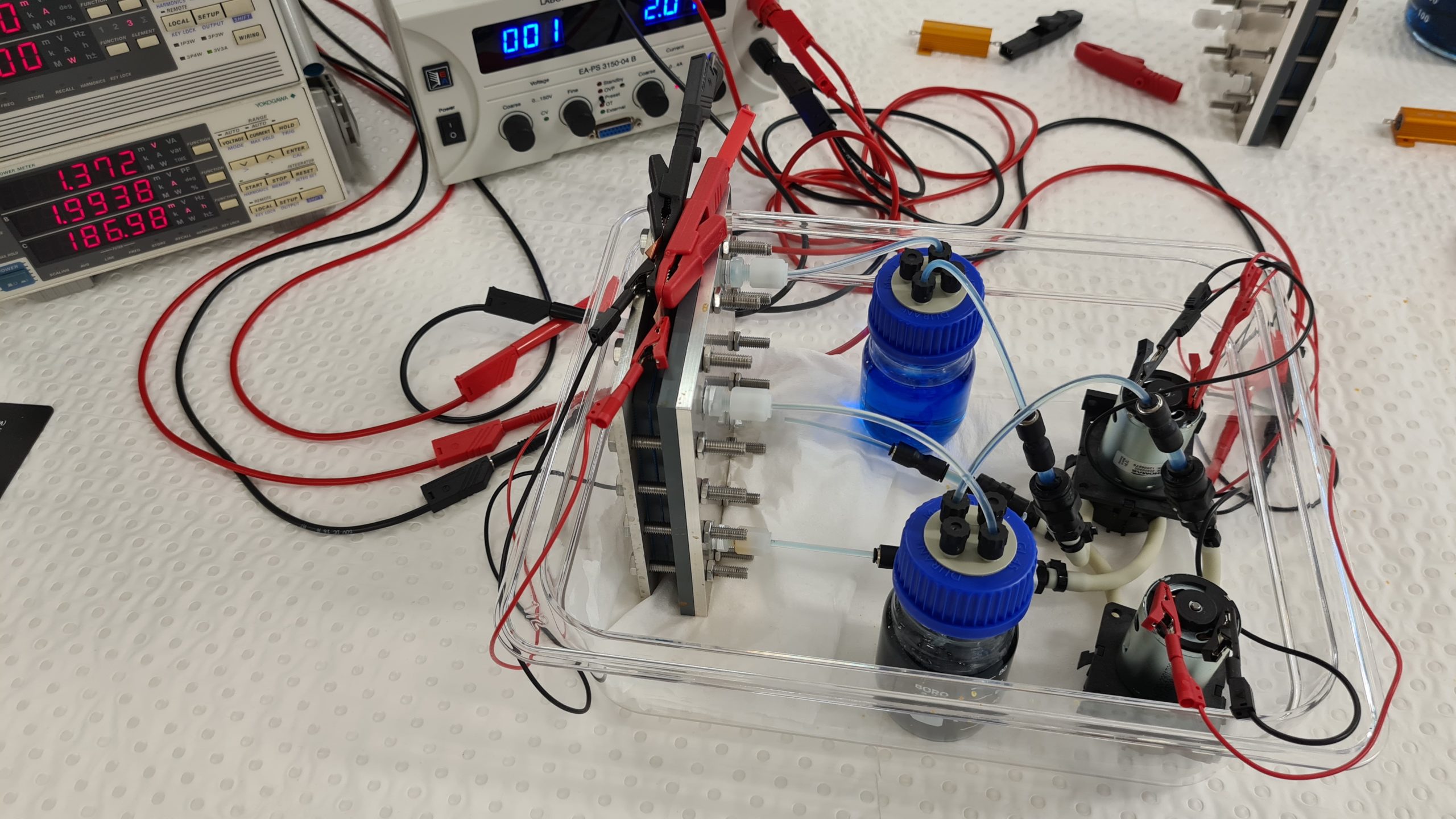

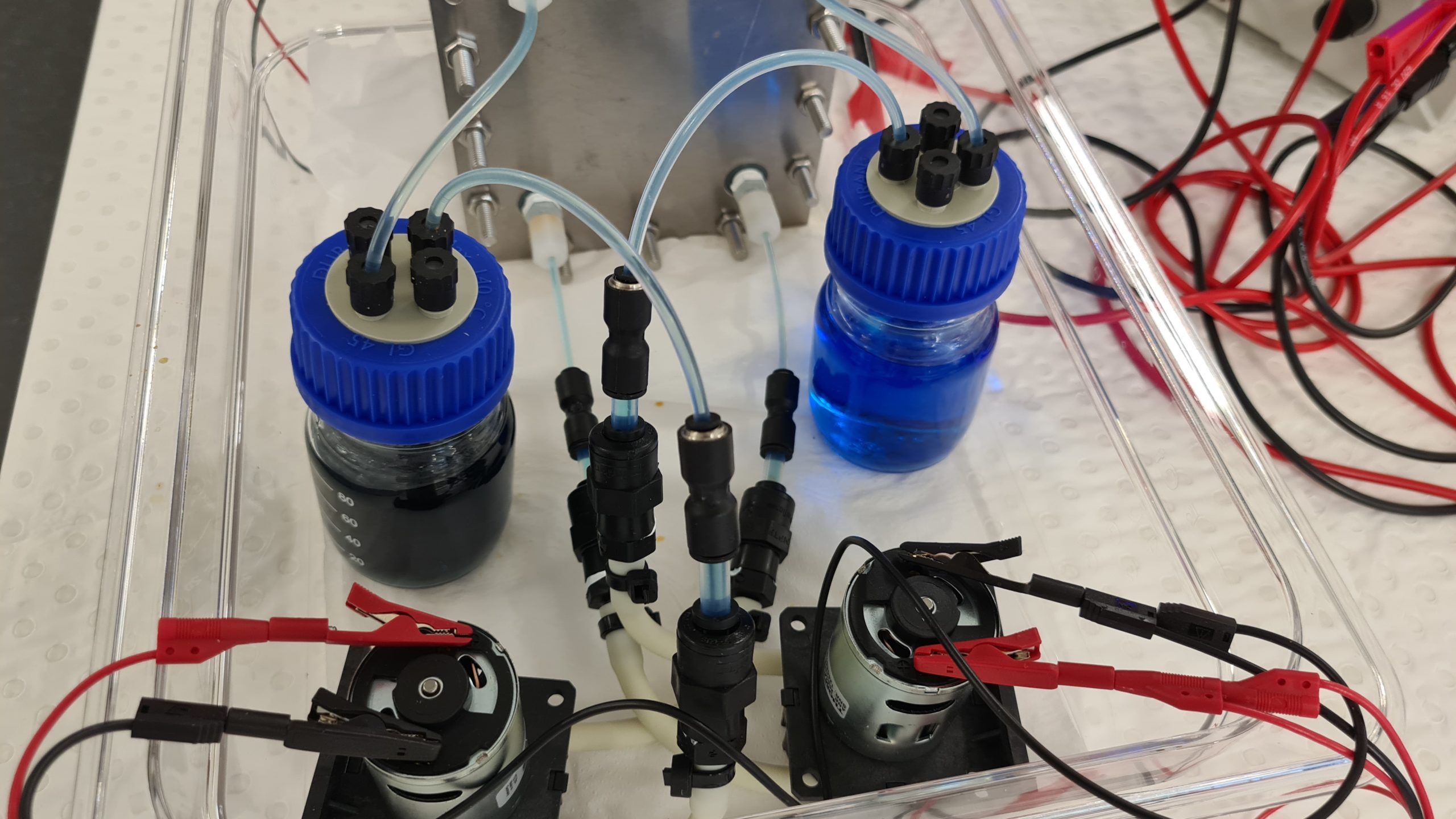
Setting up a Fe/Fe RFB for teaching purposes at UNSW.
Here is an iron/iron redox flow battery set up for educational purposes. A Fe/Fe-RFB uses a green Fe(II) salt solution as the initial solution in the discharged state, whereby the solution is only slightly acidic. During the charging process, more brown iron(III) ions are formed in the positive solution. The negative solution becomes paler because the concentration of iron(II) ions in the solution decreases with the state of charge due to the deposition of elemental iron. As a result, the state of charge can also be detected very well here.

Talks about collaborations at the University of Technology Sydney
Today I met with the Dean of International Cooperation at UTS to discuss possible joint activities in collaboration with Fraunhofer and the University of New South Wales. We will undertake further reciprocal visits in the future and expand our joint activities.





